g to atoms|atoms to mass in grams : Bacolod The procedure to use the grams to atoms calculator is as follows: Step 1: Enter the atomic mass number, grams and x in the respective input field. Step 2: Now click the button . web1 de fev. de 2024 · Getafe e Real Madrid se enfrentam nesta quinta-feira (1), às 17h (Brasília), no Coliseum Alfonso Pérez, pela 22ª rodada de LALIGA. O duelo terá .
0 · how to turn grams atoms
1 · how to convert g atoms
2 · g to atoms calculator
3 · converting from atoms to mass
4 · atoms to mass in grams
5 · atoms to grams chemistry
6 · atoms to gram calculator
7 · atoms to g formula
8 · More
WEB23 de abr. de 1982 · IMDb RATING. 7.2 /10. 14K. YOUR RATING. Rate. Play trailer 1:20. 2 Videos. 99+ Photos. Crime Music Thriller. Two tapes, two Parisian mob killers, one .
g to atoms*******The procedure to use the grams to atoms calculator is as follows: Step 1: Enter the atomic mass number, grams and x in the respective input field. Step 2: Now click the button .Learn how to calculate the number of atoms in a substance from its mass using molar mass, moles, and Avogadro's number. See examples and formulas for atoms, molecules, and ions.This all-in-one online Grams to Atoms Calculator performs calculations using a formula that relates the mass of a substance in grams to the number of atoms (or molecules) in the .This is the value that you want to convert to atoms. Multiply the number of grams by Avogadro’s number: grams * N = atoms. Divide the result from step 3 by the atomic . This chemistry video tutorial explains the conversion process of grams to atoms which is useful in solving common stoichiometry practice problems. This vide.
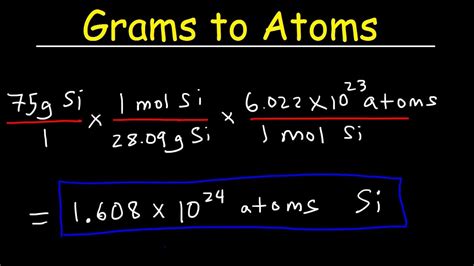
16.3 g / (32.06 g/mol) = 0.508 moles. 0.508 moles * (6.022 x 10^23 atoms/mol) = 3.064 x 10^23 atoms. Therefore, there are approximately 3.064 x 10^23 atoms in 16.3 grams of .
Grams To Atoms Formula: Demystifying the Calculation . When dealing with chemical compositions, understanding the conversion from grams to atoms is pivotal. The formula . To calculate this result: Calculate the molar mass of water, which is two hydrogen atoms' and one oxygen atom's molar masses combined: (2 × 1.008 g/mol) + .

Let’s walk through an example to illustrate how the Atoms ↔ Grams Calculator works: Suppose you have a sample of hydrogen gas with 6.02 x 10^23 atoms, and you know . This chemistry video tutorial explains the conversion process of grams to atoms which is useful in solving common stoichiometry practice problems. This vide.
So, to find the number of hydrogen atoms in a mole of water molecules, the problem can be solved using conversion factors: 1mol H2O × 6.02 ×1023 moleculesH2O 1 molH2O × 2atoms H 1 moleculeH2O = 1.20 ×1024 atomsH 1 mol H 2 O × 6.02 × 10 23 molecules H 2 O 1 mol H 2 O × 2 atoms H 1 molecule H 2 O = 1.20 × 10 24 atoms H.g to atoms How to Convert Atom to Gram? Determine the Molar Mass: Find the molar mass of the substance in grams per mole (g/mol). This information is typically available on the periodic table. Count the Number of Atoms: Determine the number of atoms you have. Use Avogadro's Number: Utilize Avogadro's number, which is approximately \(6.022 .Grams To Atoms Formula: Demystifying the Calculation . When dealing with chemical compositions, understanding the conversion from grams to atoms is pivotal. The formula for this conversion can be expressed mathematically using the Grams To Atoms Formula:
The conversion factors are: To convert the moles of Cl – to number of Cl – ions, we use the equivalence, 1 mole Cl – = 6.02 x 10 23 Cl – ions. The conversion factors are: In 6.75 grams of MgCl 2, there are 8.54 x 10 22 chloride ions. When asked how many ions, atoms, molecules, etc., use Avogadro’s number.
Grams ( Grams ): 100. Atomic Mass ( Atomic Mass ): 63.546 g/mol (Copper) Using the formula: Atoms = ( 100 63.546) × 6.022 × 10 23. Calculating: Atoms ≈ 9.52 × 10 23. These examples demonstrate the versatility of the Grams To Atoms Formula in converting grams of various substances into their respective atomic quantities. Input the Average Atomic Mass: Enter the average atomic mass of the substance in atomic mass units (amu). Choose the Conversion: Click on “Convert Atoms to Grams” for converting atoms to grams, or “Convert Grams to Atoms” for the reverse conversion. View the Result: The result will be displayed in the result section. Solutions to Example 6.2.2, Summing the molar masses of the atoms in the NaCl formula unit gives. 1 Na molar mass: 22.99 g. 1 Cl molar mass: 35.45 g. Total: 58.44 g. The mass of 1 mol of NaCl is 58.44 g. Multiplying the molar mass of each atom by the number of atoms of that type in bilirubin’s formula and adding the results, we get.g to atoms atoms to mass in grams To calculate the number of atoms in a sample, divide its weight in grams by the amu atomic mass from the periodic table, then multiply the result by Avogadro's number: 6.02 x 10^23. Express the relationship of the three pieces of information you need to calculate the number of atoms in the sample in the form of an equation.
The Wikipedia article on the mole states: The term gram-atom (abbreviated gat.) has been used for a related but distinct concept, namely a quantity of a substance that contains Avogadro's number of atoms, whether isolated or combined in molecules. Thus, for example, 1 mole of MgBX2 M g B X 2 is 1 gram-molecule of MgBX2 M g B X 2 but 3 .The molar mass is expressed in grams per mole (g/mol) and represents the mass of one mole of a substance. To convert grams to atoms, use the formula: Number of Atoms or Molecules = Mass (g) / Molar Mass (g/mol) ×Avogadro’s Number; This equation allows the calculation of the number of atoms or molecules corresponding to a given mass of a .
Resultados de Exames? Clique aqui! Este sistema utiliza coo.
g to atoms|atoms to mass in grams